BACKGROUND
CD19 chimeric antigen receptor (CAR) T-cell therapies have transformed the treatment landscape for patients (pts) with relapsed/refractory B-cell malignancies, but they remain limited by significant toxicities: cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). While a more favorable toxicity profile had been reported with lisocabtagene maraleucel (liso-cel), based on our real-world experience CRS and ICANS are still observed in 70% and 30% of pts, respectively (Portuguese et al., ASH abstract #172978). Data from our group (Gazeau, TCT, 2023) and others (Strati, Blood, 2023; Park, Nat Med, 2023) suggested the recombinant interleukin-1 receptor antagonist anakinra may successfully prevent and treat CRS and ICANS. Thus, we conducted a phase 2 investigator-initiated clinical trial of anakinra to prevent CRS and ICANS after liso-cel (NCT04359784). We report here our planned interim analysis (n = 15).
METHODS
Adults ≥18 years of age with Karnofsky performance status ≥60% and B-cell non-Hodgkin lymphoma receiving liso-cel per the FDA label were eligible for this study. Anakinra was administered at 200 mg/day SC from day 0 through day +13 after liso-cel infusion, with the first dose administered approximately 2-4 hours prior to liso-cel infusion. CRS and ICANS severity were assessed per 2019 ASTCT criteria. The primary endpoint was the absence of any grade CRS assessed using the Bayesian optimal phase 2 design.The primary endpoint was not met if ≥6 of the first 15 pts developed any grade CRS.
RESULTS
Nineteen pts were screened for the study and 15 pts were enrolled. All 15 pts received liso-cel and all doses of SC anakinra. All pts were evaluable for toxicity assessment; 12 pts were evaluable for disease response assessment at day +28 (n = 3, no measurable disease prior to lymphodepletion [LD]).
Pt and disease characteristics are shown in Table 1. Eleven pts (73%) had diffuse large B-cell lymphoma, 3 (20%) had active CNS involvement, and 5 (33%) had bulky disease (≥5 cm). Median pre-LD serum ferritin and C-reactive protein (CRP) were 153 ng/mL (IQR, 129-231) and 6.8 (IQR, 1.5-17.2), respectively.
All 15 pts received anakinra as planned without dose adjustment or interruption. There were no injection-site reactions or AEs attributed to anakinra. Two pts developed infections during anakinra treatment and were both due to mild Clostridium difficile colitis requiring oral antibiotics.
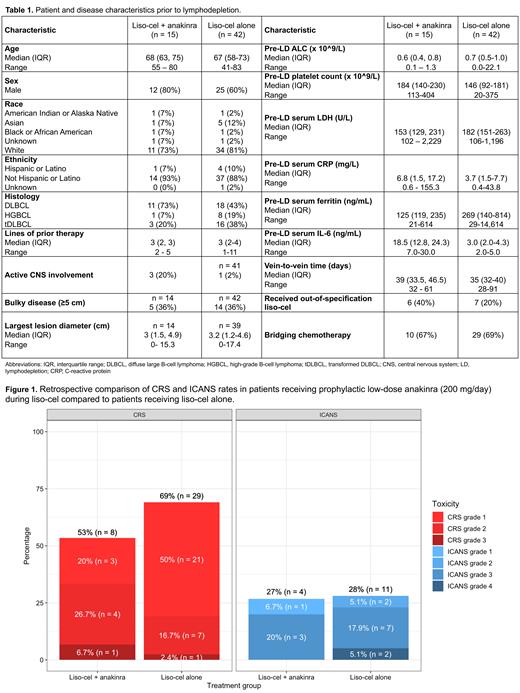
Eight pts (53%) developed any grade CRS, including 1 (7%) case of grade 3 CRS. Four pts (27%) developed any grade ICANS, including 3 (20%) with grade 3 (Figure 1). Median CRS onset and duration were 2 and 2.5 days, respectively, and median ICANS onset and duration were 6 and 9 days, respectively. Three pts (20%) required tocilizumab (range, 0-1 doses), 4 (50%) required dexamethasone (median total dose, 5 mg; range, 0-200 mg), and 1 (12%) required high-dose methylprednisolone. The best overall response rate (ORR) by Lugano criteria was 67% (complete response, n = 5 [42%]).
Study pts were retrospectively compared to pts who received liso-cel per standard of care off trial without prophylactic anakinra at our center (n = 42). Despite higher median pre-LD CRP and IL-6 levels, the incidence of CRS was lower in anakinra-treated compared to anakinra-untreated pts (53% vs. 69%, respectively) with comparable median duration of CRS (2.5 vs. 3 days, respectively). The incidence and duration of ICANS were similar (27% vs. 28%; median 9 vs. 8 days, respectively), as was the incidence and duration of hospitalization (67% vs. 74%, median 9.5 vs. 10 days, respectively). Based on Locally Estimated Scatterplot Smoothing (LOESS) estimates, time from liso-cel infusion to CRP normalization was faster in patients receiving anakinra (9 days) compared to those receiving liso-cel alone (14 days).
CONCLUSION
Low-dose SC anakinra prophylaxis during liso-cel treatment was very well tolerated with preserved anti-tumor efficacy. While our primary endpoint was not reached at our planned interim analysis, we observed lower CRS rates and faster resolution of CRS in comparison to a real-world cohort of pts receiving liso-cel without prophylactic anakinra. To further enhance the preventative effects of anakinra, we plan to change the administration route from SC to intravenous, and to allow anakinra dose uptitration for the last 10 participants in the study.
OffLabel Disclosure:
Newell:Immunoscape: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Neogene Therapuetics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Nanostring Technologies: Membership on an entity's Board of Directors or advisory committees. Shadman:AbbVie, Genentech, AstraZeneca, Pharmacyclics, BeiGene, BMS, MorphoSys/Incyte, Kite, Eli Lilly, Genmab, Mustang Bio, Regeneron, ADC therapeutics, Fate Therapeutics, Janssen and MEI Pharma: Consultancy; Mustang Bio, BMS, Pharmacyclics, Genentech, AbbVie,TG Therapeutics, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, Vincerx: Research Funding. Hirayama:Bristol Myers Squibb: Honoraria, Research Funding; Juno Therapeutics, a Bristol Myers Squibb Company: Research Funding; Nektar Therapeutics: Honoraria, Research Funding; Novartis: Honoraria. Till:BMS/Juno Therapeutics: Research Funding; Mustang Bio: Consultancy, Patents & Royalties, Research Funding; Proteios Technology: Consultancy, Current holder of stock options in a privately-held company. Kimble:Juno/BMS: Research Funding. Iovino:Mustang Bio: Current equity holder in publicly-traded company. Chapuis:Juno Therapeutics: Research Funding. Cassaday:Amgen: Consultancy, Honoraria, Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Merck: Research Funding; PeproMene Bio: Membership on an entity's Board of Directors or advisory committees; Autolus: Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Vanda Pharmaceuticals: Research Funding; Servier: Research Funding; Seagen: Other: Spouse was employed by and owned stock in Seagen within the last 24 months.. Milano:ExCellThera Inc.: Research Funding. Maloney:Legend Biotech: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Other: Member of the Scientific Advisory Board; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board , Research Funding; Genentech: Consultancy, Honoraria, Other: Chair and Member of the Lymphoma Steering Committee; MorphoSys: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Other: Member of the JCAR017 EAP-001 Safety Review Committee and Member, CLL Strategic Council, Member of the JCAR017-BCM-03 Scientific Steering Committee under BMS, Research Funding; Kite, a Gilead Sciences: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: Rights to royalties from Fred Hutch for patents licensed to Juno Therapeutics/BMS, Research Funding; Janssen: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Navan Technologies: Consultancy, Honoraria, Other: Member of the Scientific Advisory Board; Navan Technologies: Current holder of stock options in a privately-held company; Chimeric Therapeutics: Other: Member of the Scientific Advisory Board; Gilead Sciences: Consultancy, Honoraria, Other: Member, Scientific Review Committee, Research Scholars Program in Hematologic Malignancies; Umoja: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Fred Hutch: Other: rights to royalties for patents licensed to Juno; Bioline Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board ; ImmPACT Bio: Other: Member, Clinical Advisory Board, CD19/CD20 bi-specific CAR-T Cell Therapy Program; Interius: Other: Member, Clinical Advisory Board; Lyell Immunopharma: Other: Member, CAR T Steering Committee. Gauthier:Janssen: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; MorphoSys: Consultancy, Research Funding; Angiocrine Bioscience: Research Funding; Century Therapeutics: Other: Independent data review committee; Legend Biotech: Consultancy, Honoraria; Celgene (a Bristol Myers Squibb company): Research Funding; Juno Therapeutics (a Bristol Myers Squibb company): Research Funding; Sobi: Consultancy, Honoraria, Research Funding.
We are studying the safety, feasibility, and efficacy of anakinra in preventing CRS and ICANS after CD19 CAR T-cell therapy with lisocabtagene maraleucel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal